OUR CAPABILITIES
We Have You Covered Throughout Every Phase
Clinical Study Management
We offer Phase I, II and III clinical trial design, planning and execution utilizing a data-driven, flexible and efficient approach. Using innovative and precise methods for capturing and analyzing data, we identify early signals of response to put your oncology therapy on the path to approval.
Preclinical
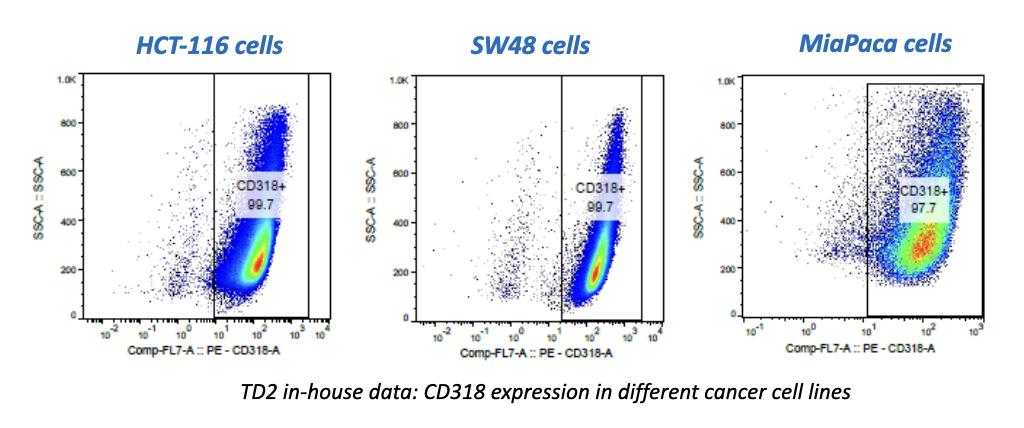
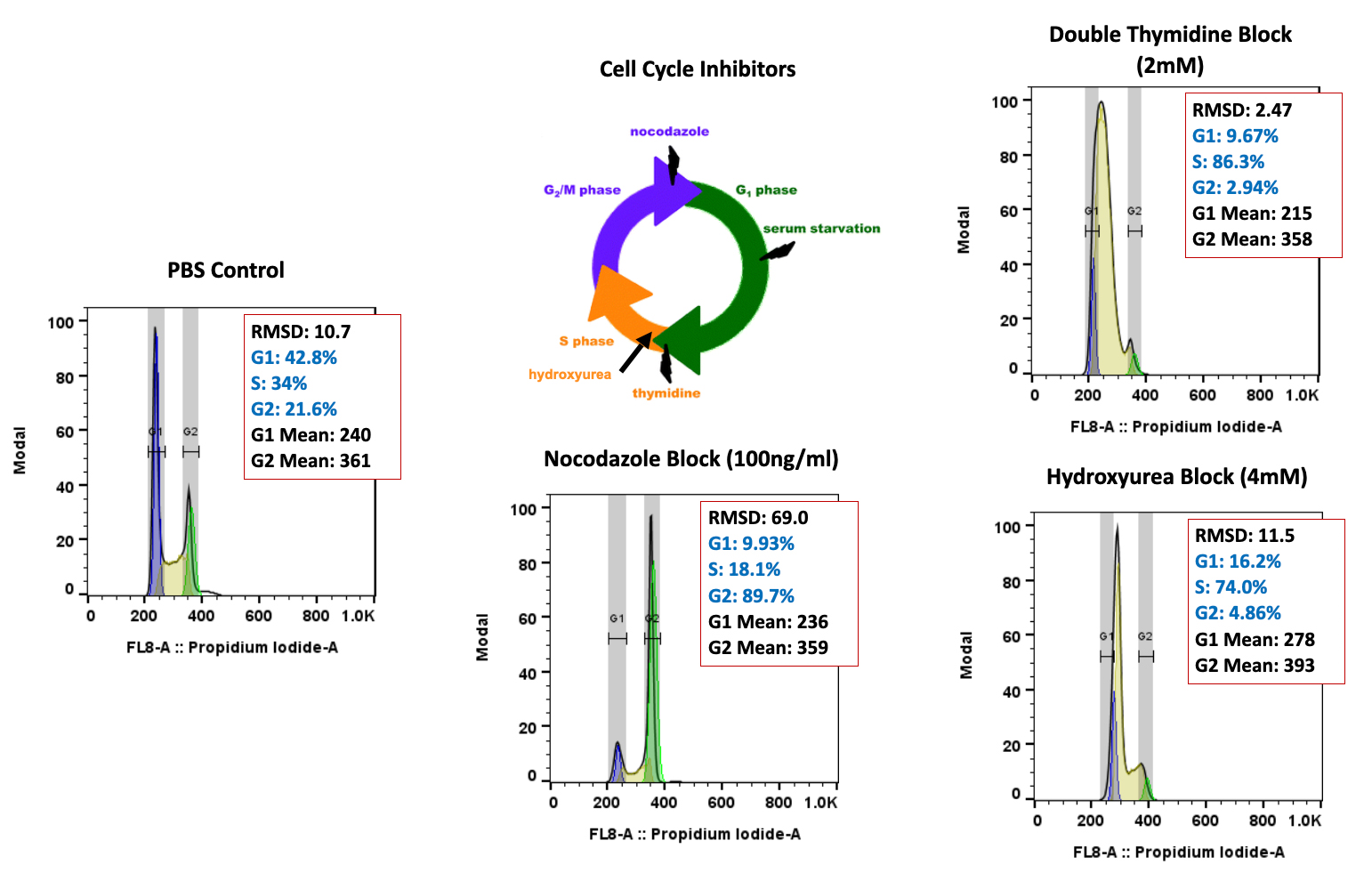
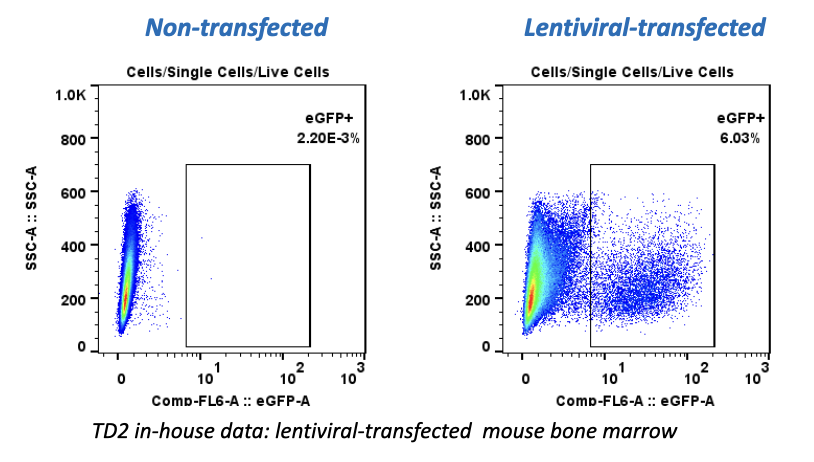
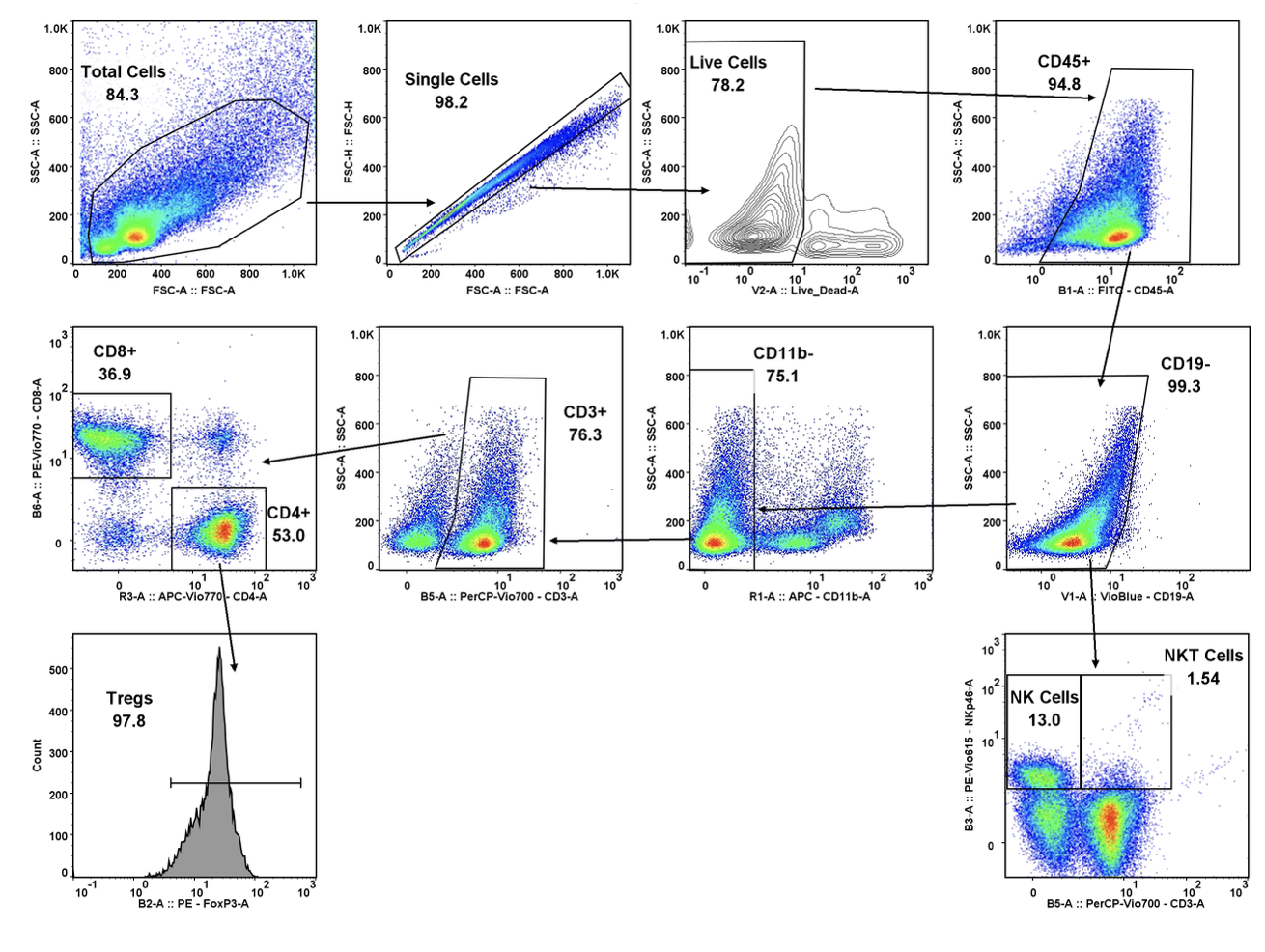
In the early phase of oncology research, TD2 sets you up for success with an extensive suite of preclinical tools, over 400 models of cancer as well as extensive knowledge in designing and adapting your study to maximize data value and predict patient response.
Regulatory Support
Your therapy’s journey to approval requires a veteran team dedicated to navigating each phase of development. At TD2, we manage the most complex aspects of the regulatory process including IND readiness assessment, program evaluation, IND development and filings, and all FDA interactions.
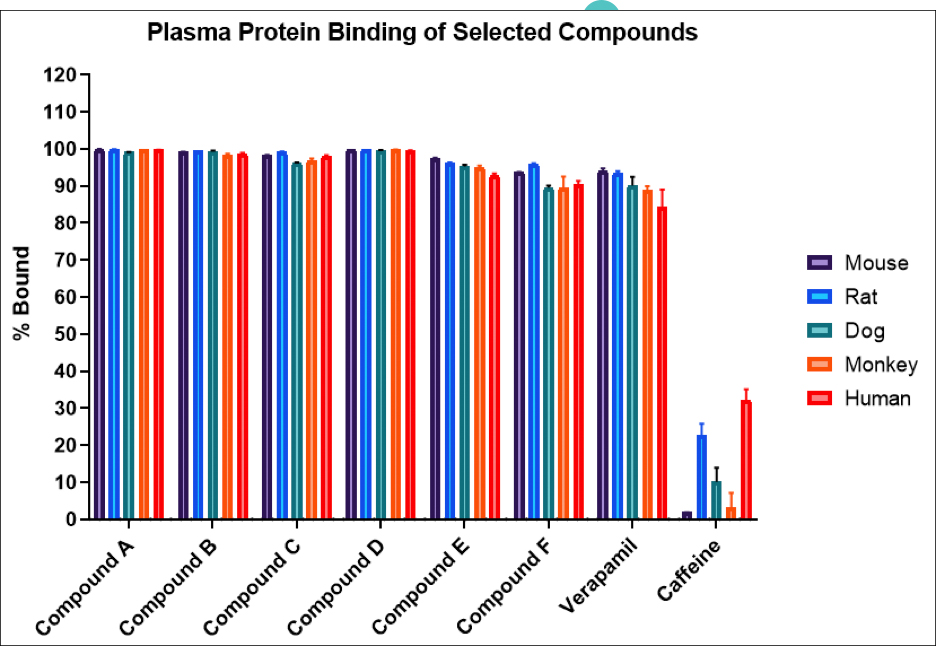
PK/ADME
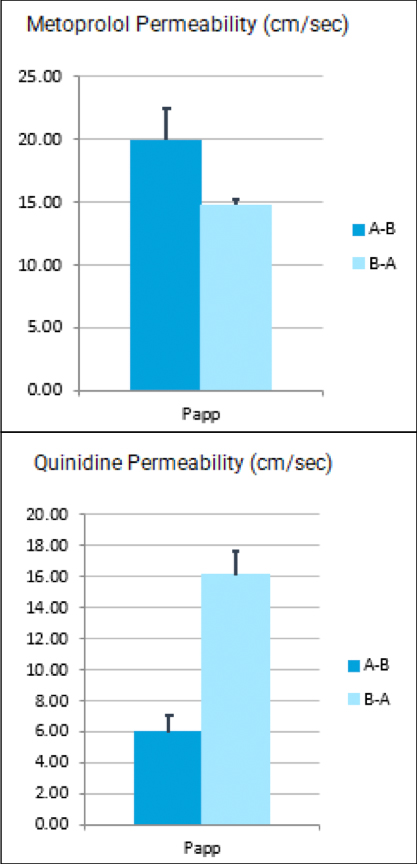
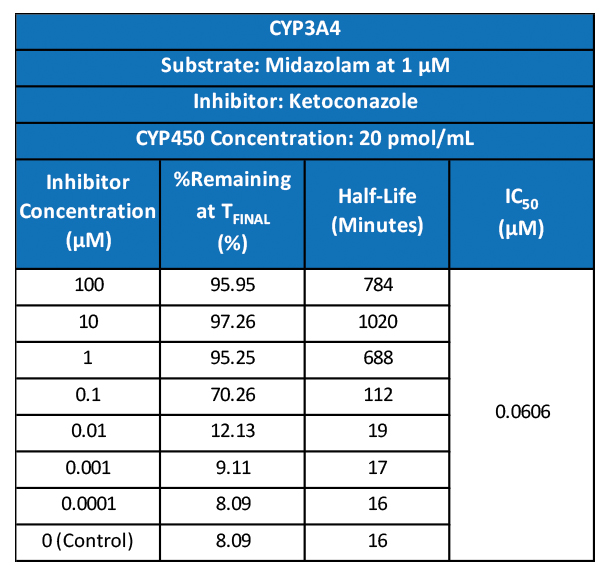
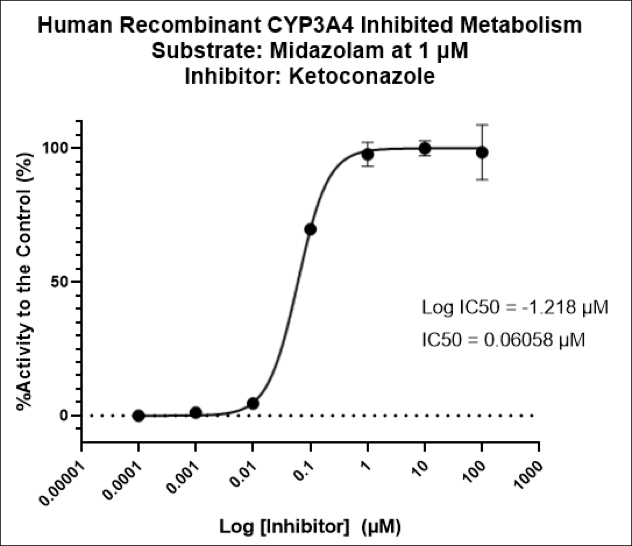
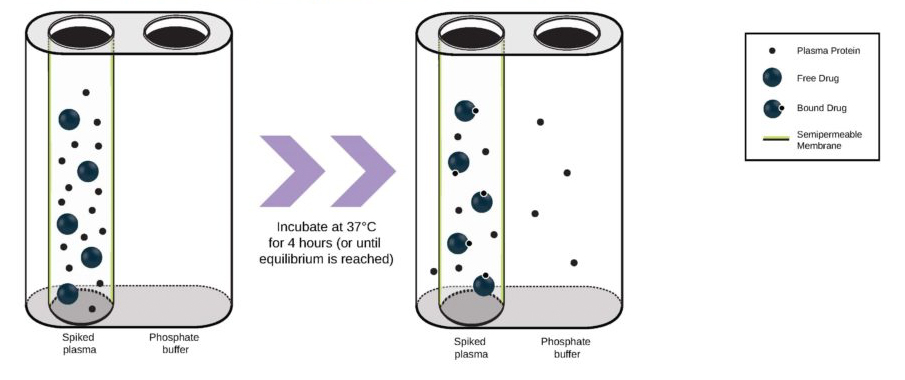
Drug metabolism and pharmacokinetic(DMPK) analyses are key to successful drug development and approval, and TD2 can help determine which tests are the best fit early through a diverse set of assays to support your program.