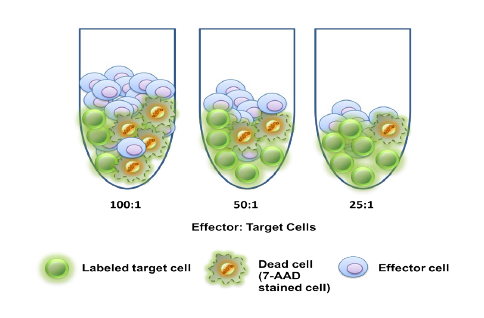
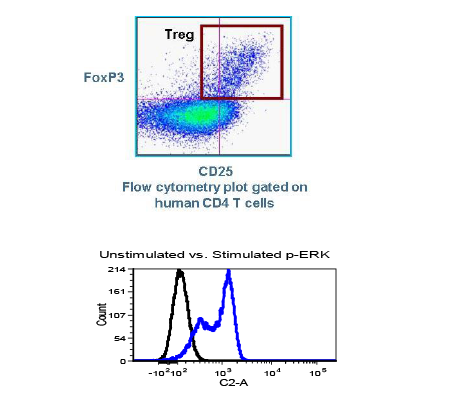
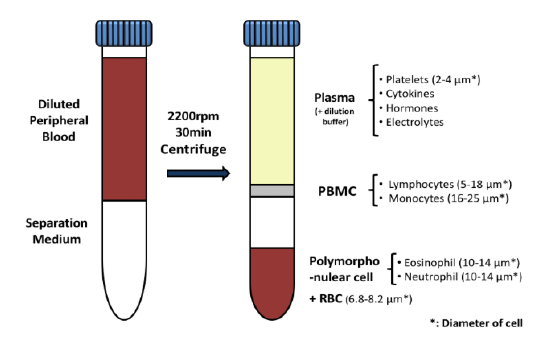
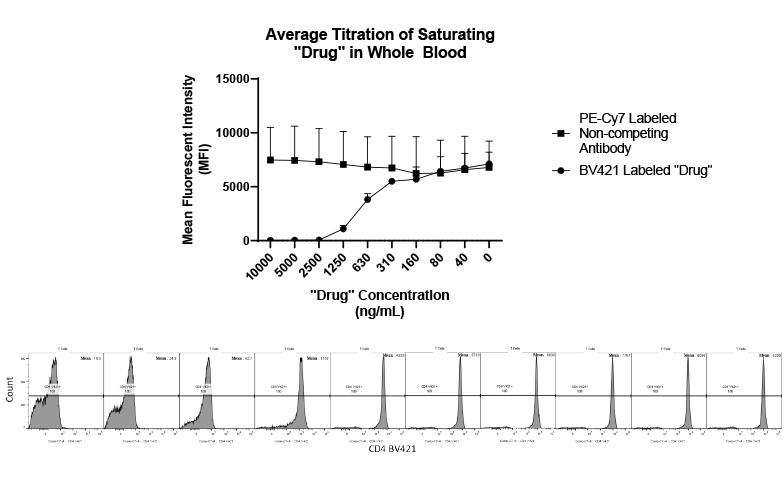
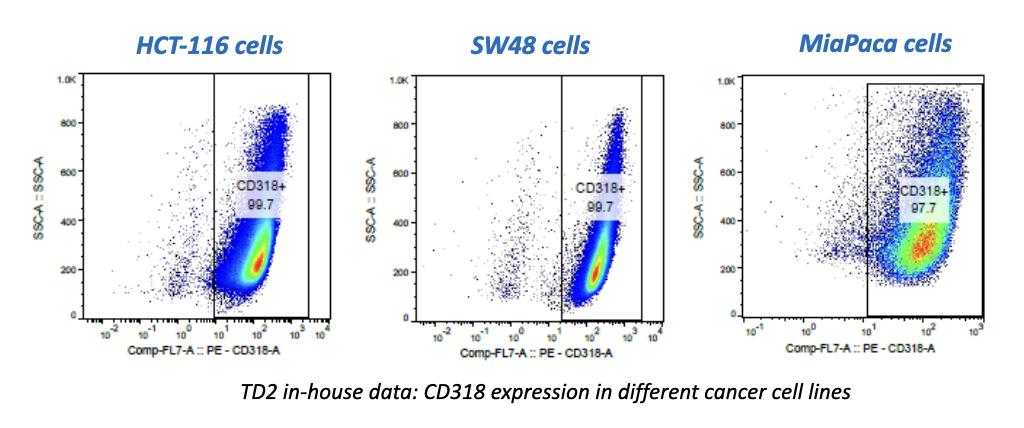
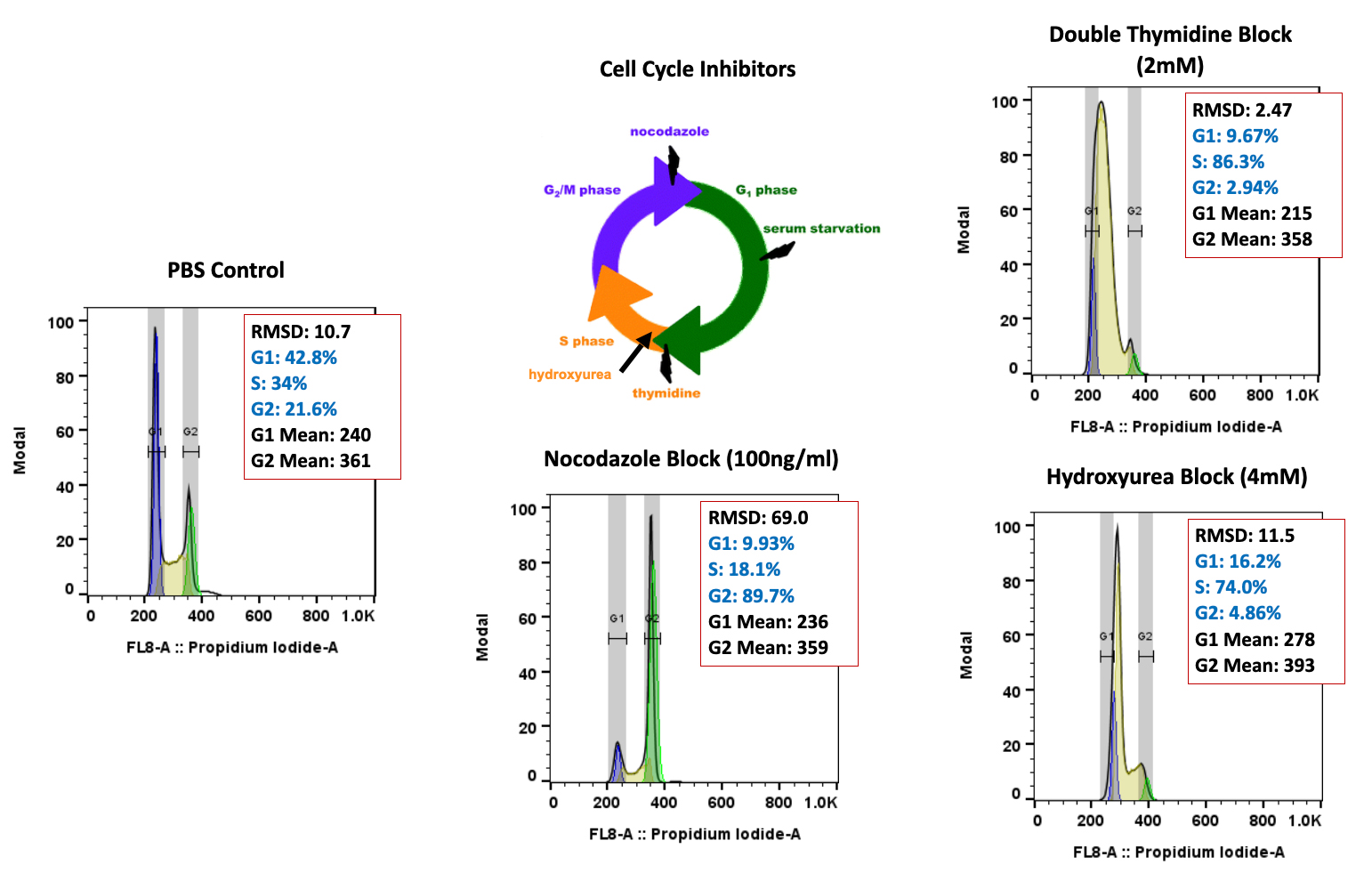
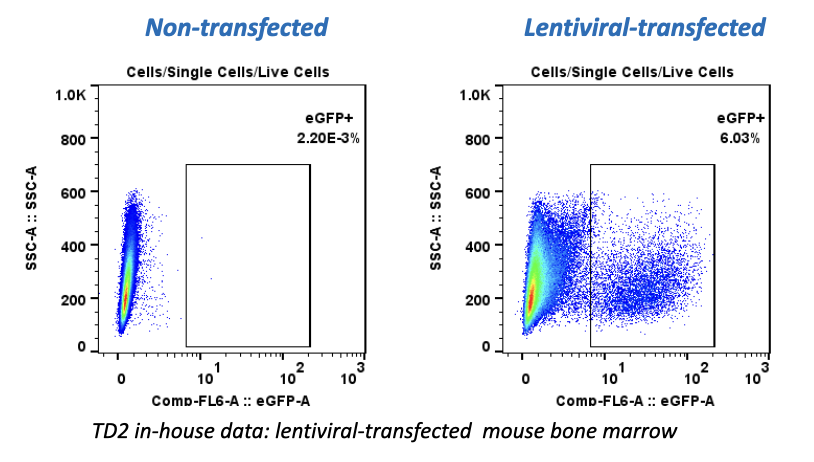
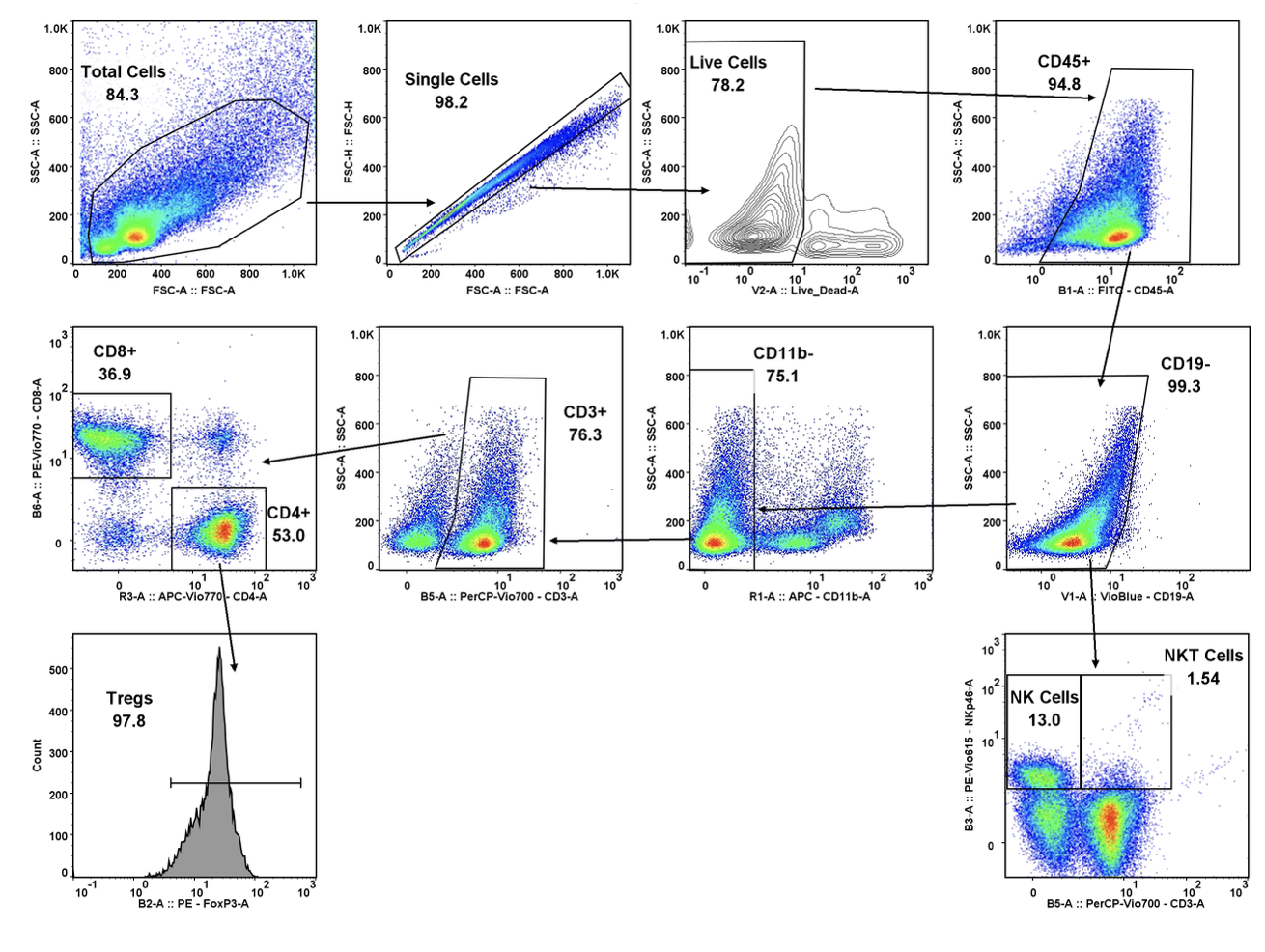
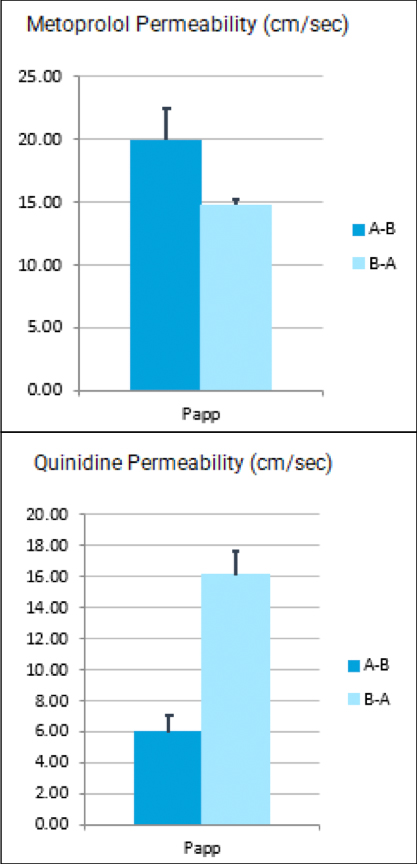
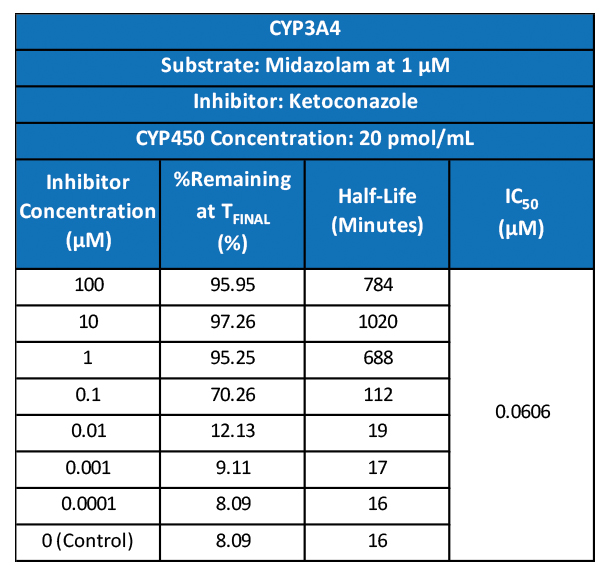
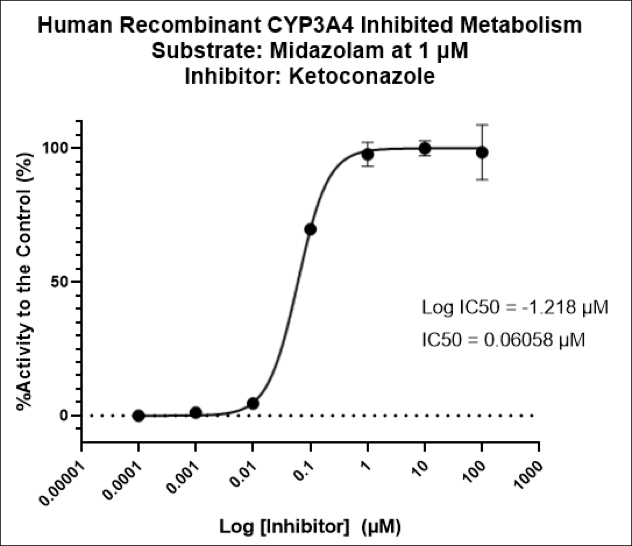
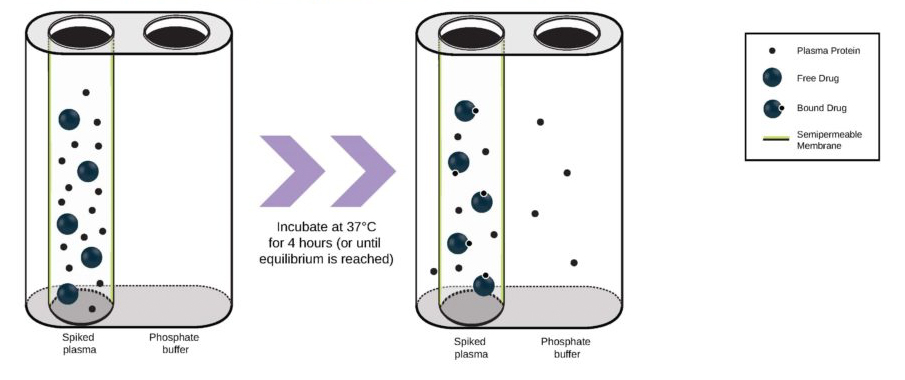
While the past decade presented us with significant biotech and pharmaceutical advances, the future of the industry looks even more promising due to a few key focus areas that are predicted to lead the market in 2020, including oncology, cell and gene therapies, and real-world evidence.
TD2 President and CEO Stephen Gately recently spoke to BioSpace about industry-defining trends, such as immuno-oncology research and traditional targeted therapies, that are anticipated to continue well into the coming years and shape the industry landscape. Not only that, according to Gately, there could be a shift in how clinical trials are organized based on economic incentives. Read the full article here to learn more.