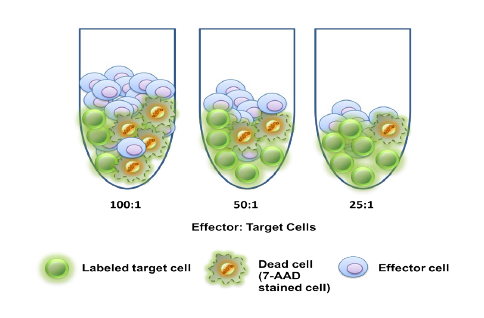
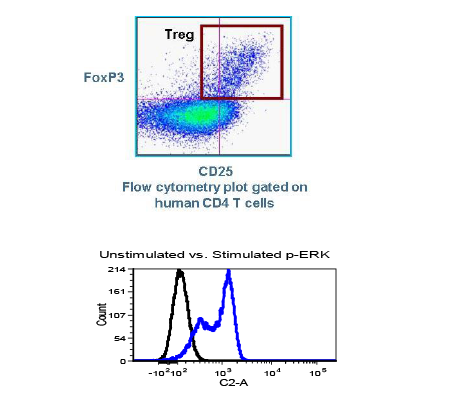
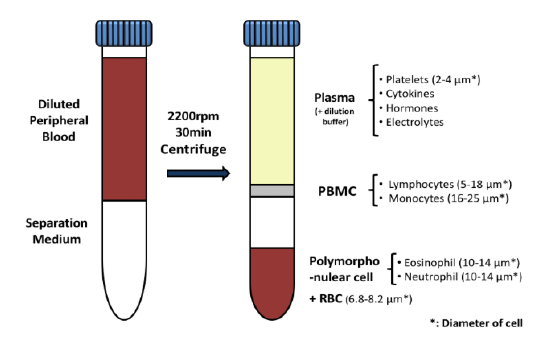
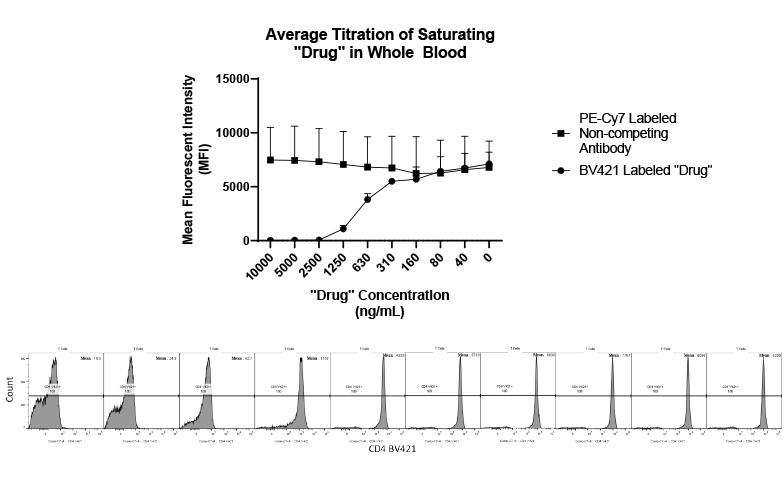
Daniel Von Hoff, M.D., Physician-in-Chief and Distinguished Professor at the Translational Genomics Research Institute (TGEN) in Phoenix will serve as the clinical trial’s principal investigator while TGen’s subsidiary, TGen Drug Development, manages this Phase 2 clinical trial on behalf of CytRx. Dr. Von Hoff was a former member of the National Cancer Advisory Board and the FDA’s Oncology Drug Advisory Committee, and former President of the American Association for Cancer Research. He currently co-directs the Pancreatic Cancer Dream Team, one of five Stand Up 2 Cancer teams comprised of renowned scientists working collaboratively on innovation, acceleration, targeted therapy and translational research in several major cancers…”
for full press release go here.