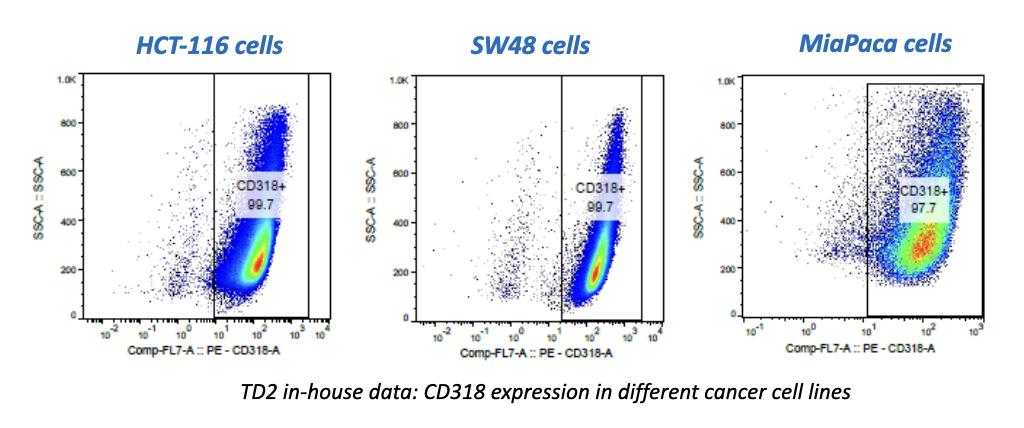
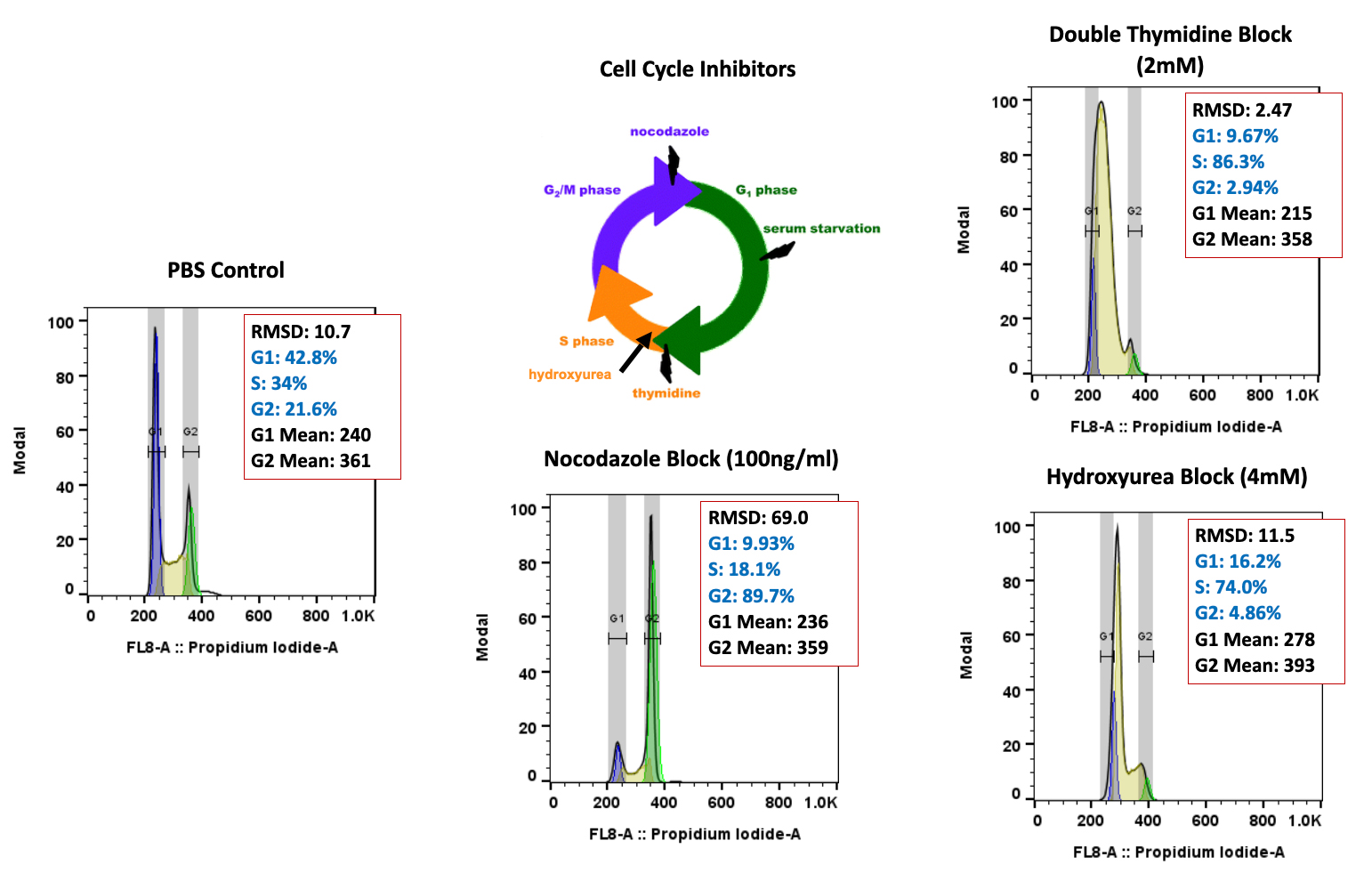
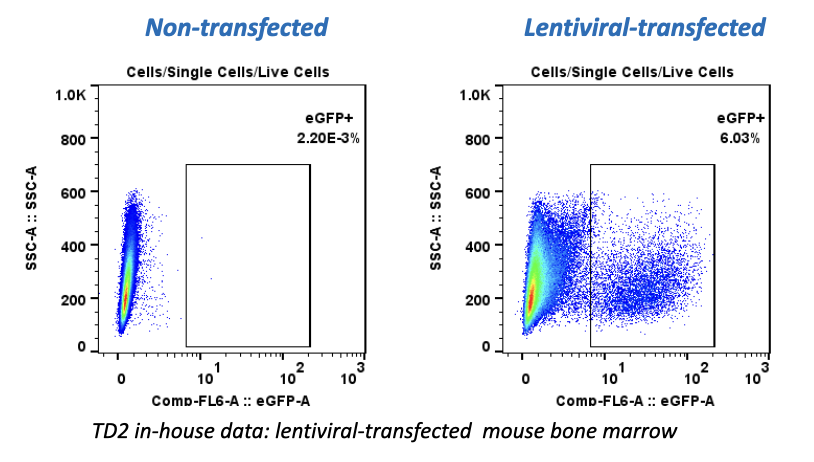
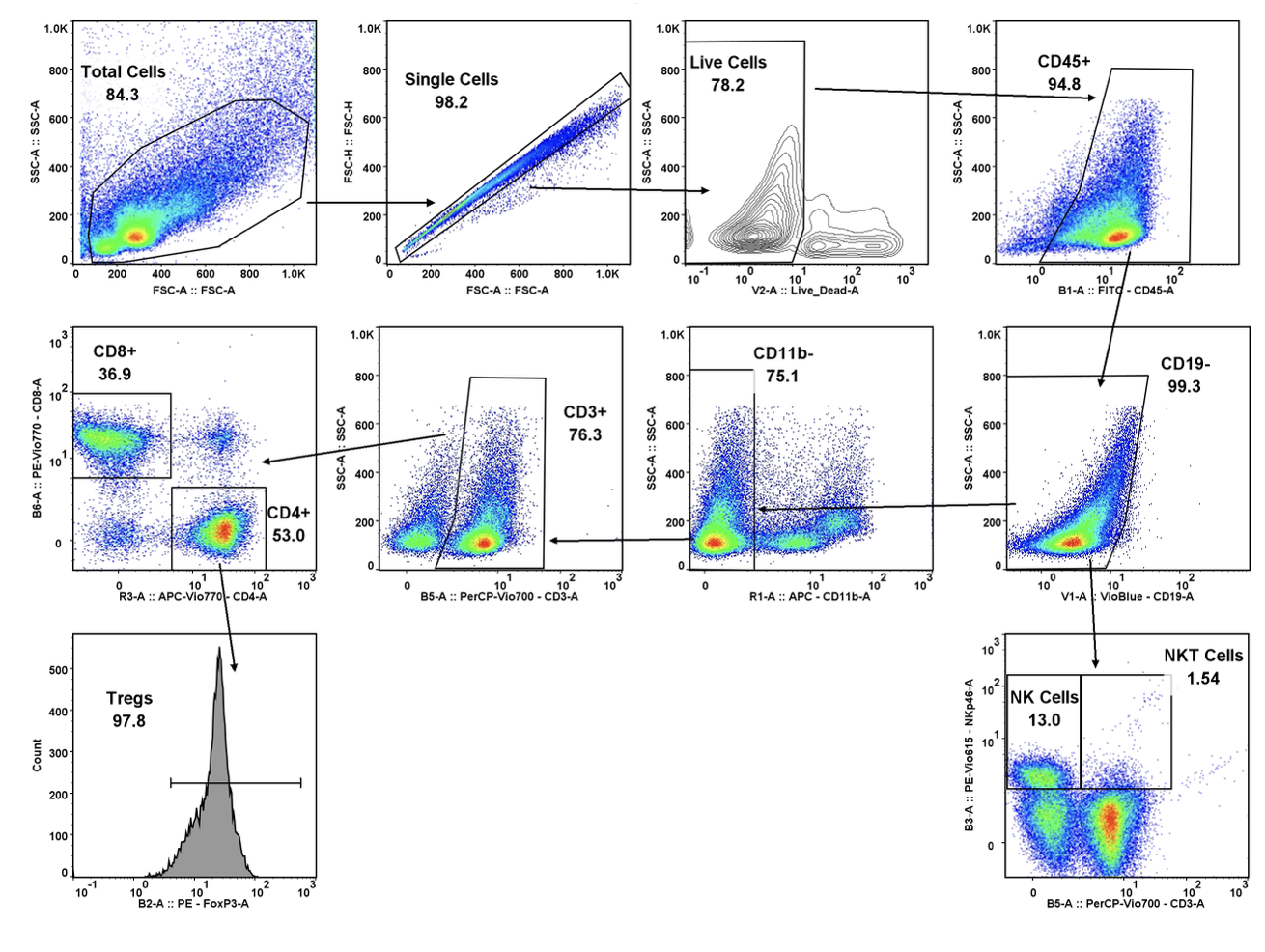
Forma Therapeutics makes deal with Celgene worth $600M
Watertown biotech firm Forma Therapeutics has announced a collaboration with drug giant Celgene Corp. potentially worth $600 million over the course of the next several years.
The partnership will not focus on any particular therapeutic area, but will use Forma’s screening technique to identify potential new drugs which can be licensed by Celgene.
The deal, which expands a previous relationship between the two companies, includes a $225 million upfront payment and an initial term of 3 ½ years, after which Celgene can opt for as many as two additional two-year terms for upfront payments totaling $375 million. If Celgene eventually opts for all three collaboration periods, it has the option to acquire Forma.
“Independent from the previously signed partnership with Celgene whereby they secured ex-U.S. rights to a defined set of protein homeostasis targets, this second agreement expands across Forma’s preclinical and future clinical development efforts, encompassing numerous protein target families and covering a broad range of therapeutic areas,” said Steven Tregay, president and CEO of Forma, in a statement. “Importantly, this new alliance enables Forma to maintain autonomy in defining our research strategy and conducting discovery through early clinical development of our product portfolio. It aligns our company with Celgene’s global leadership in hematology and immune-mediated inflammatory diseases and a shared strategic directive to transform healthcare.”
Founded in 2007, FORMA Therapeutics is focused on small-molecule cancer drugs in the areas of tumor metabolism, protein-protein interactions and epigenetics (the study of changes in gene expression or cellular phenotype). The company, which changed its name from Forma Pharmaceuticals Inc. in 2008, has secured previous deals with Novartis AG, Cubist Pharmaceuticals Inc. and The Leukemia & Lymphoma Society.