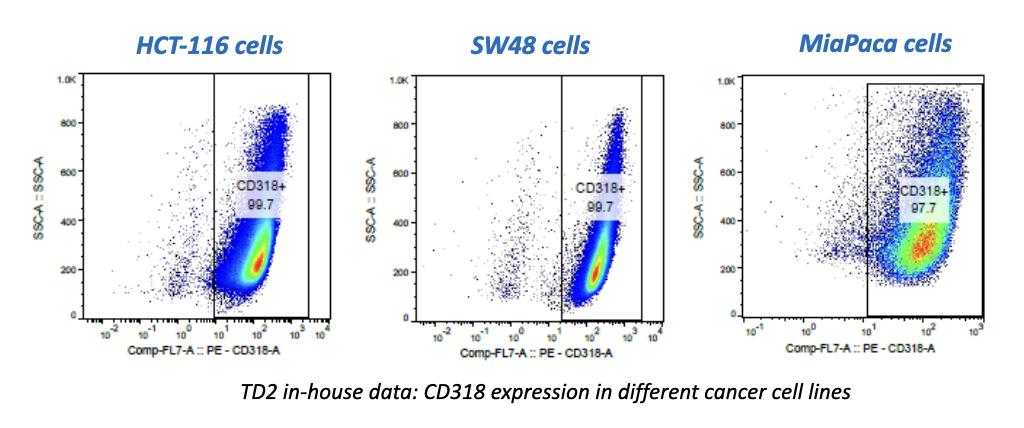
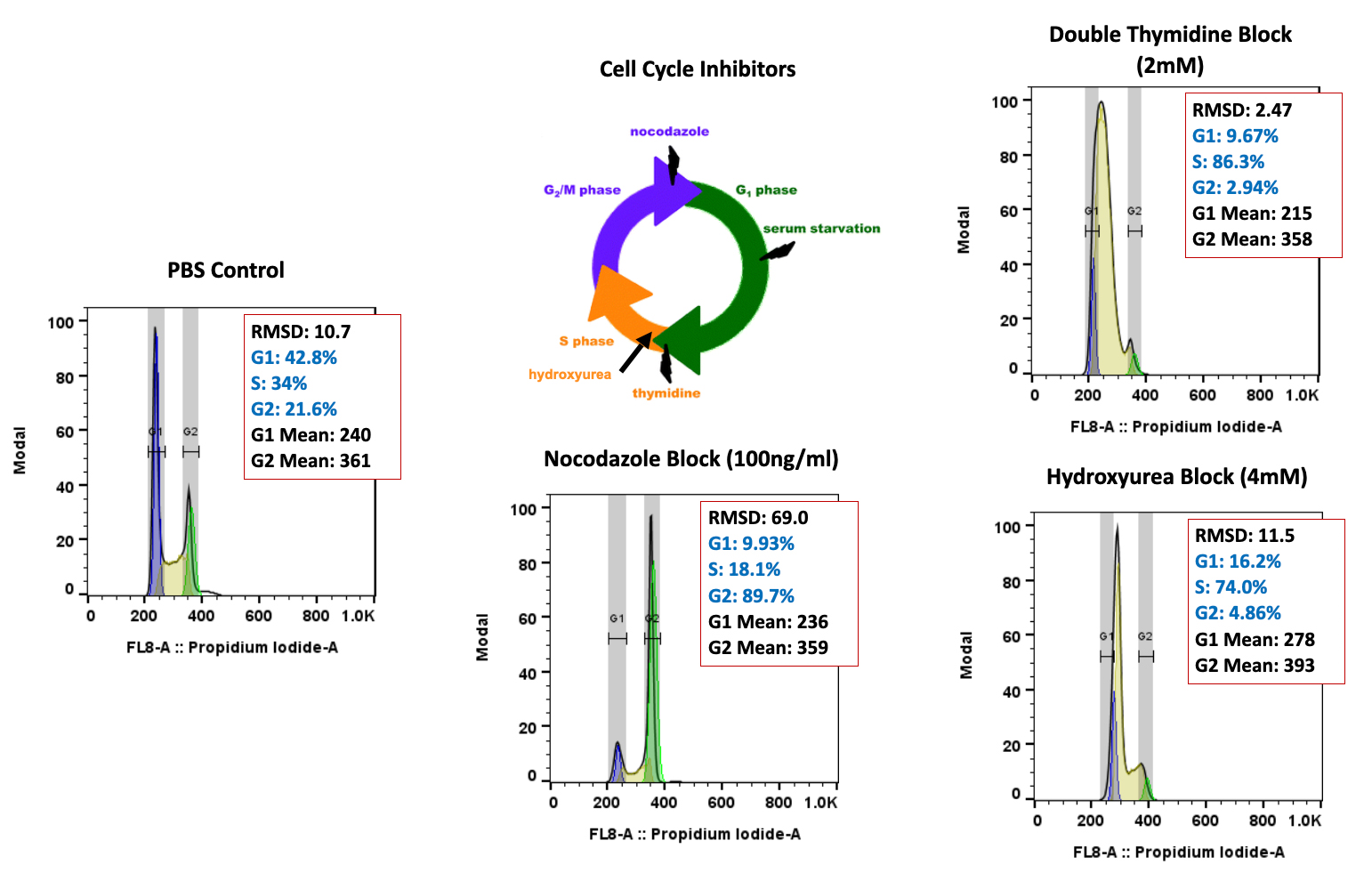
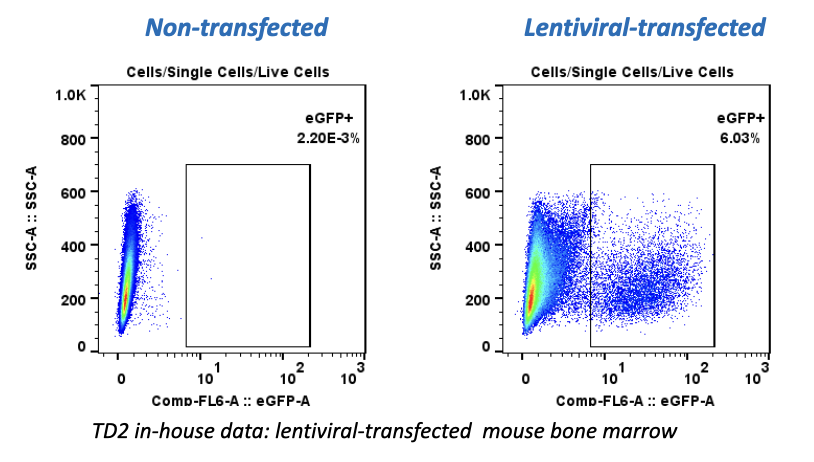
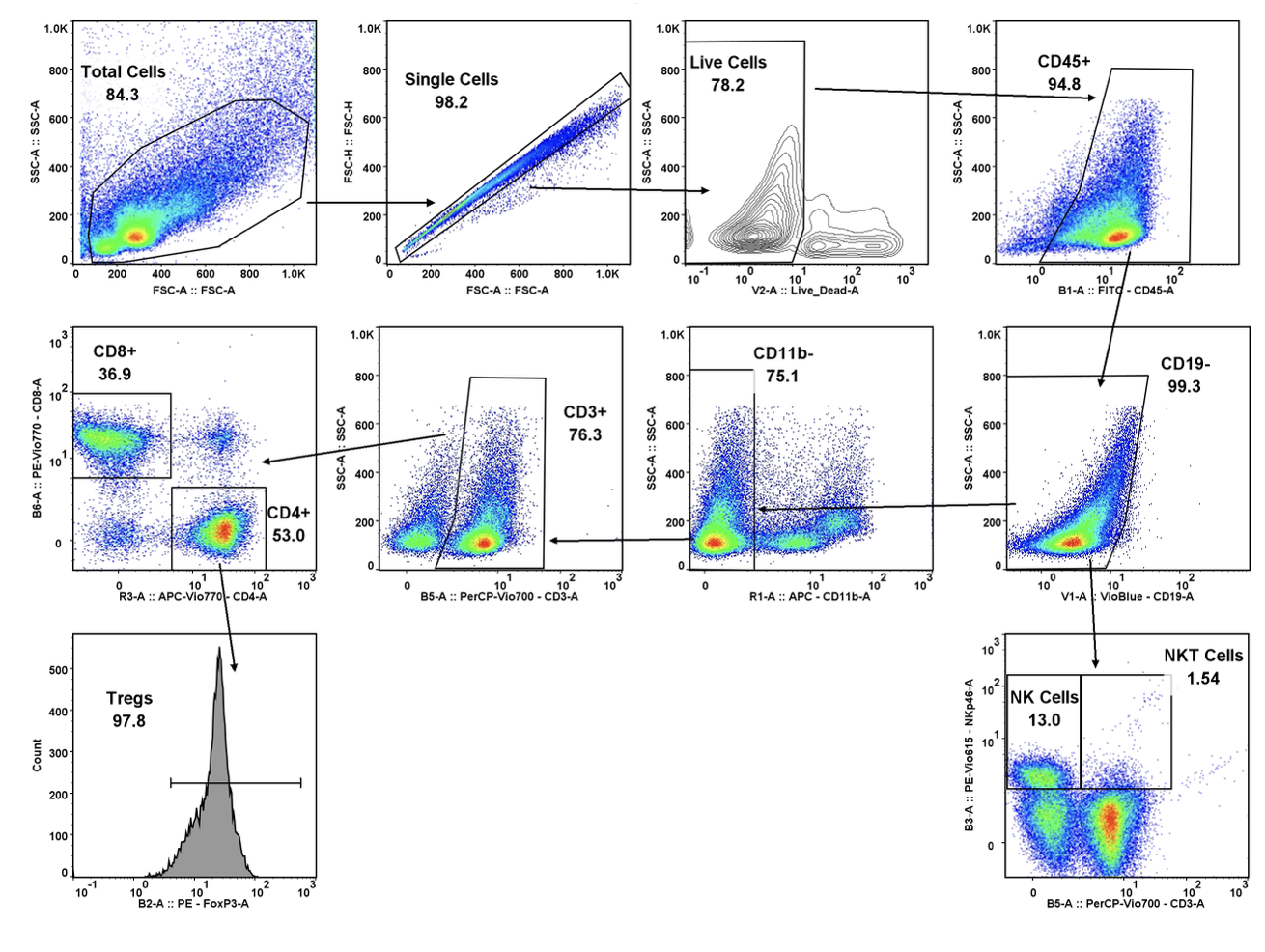
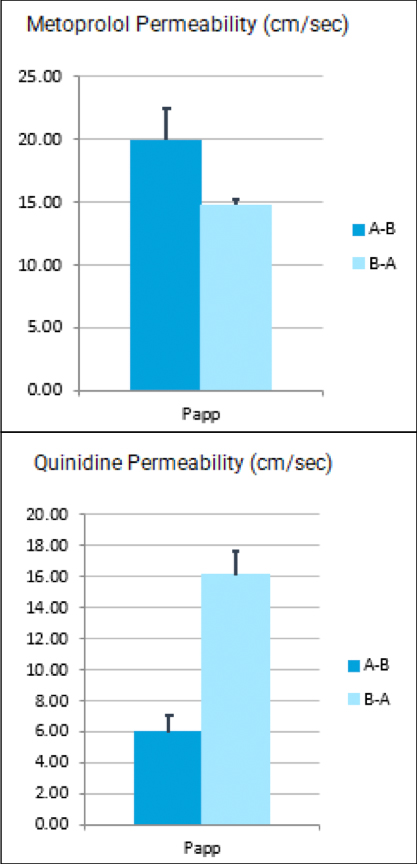
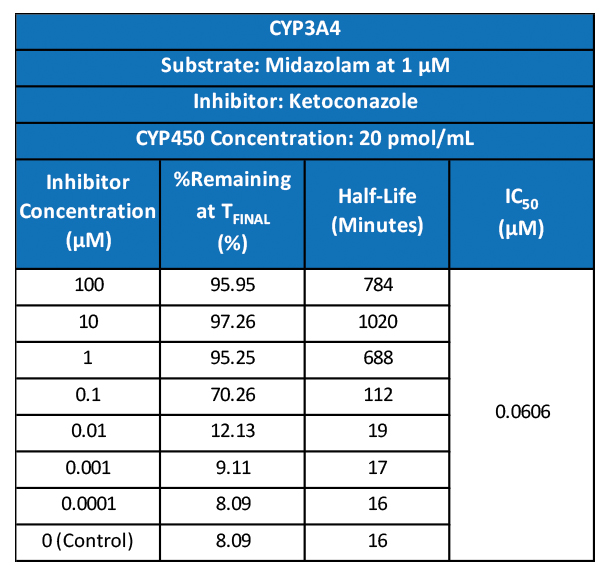
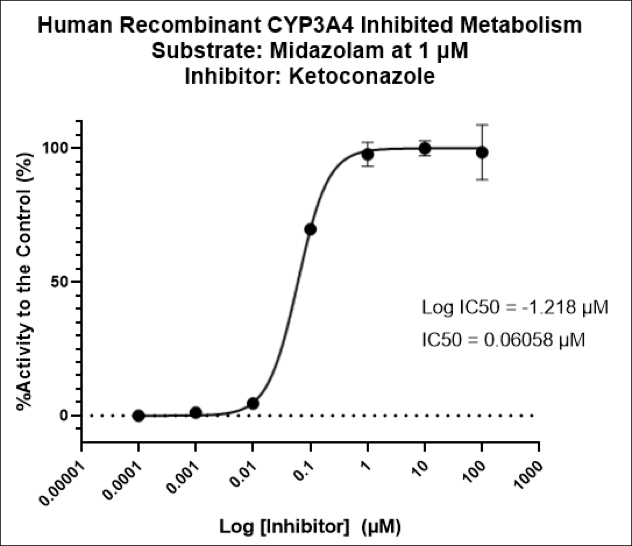
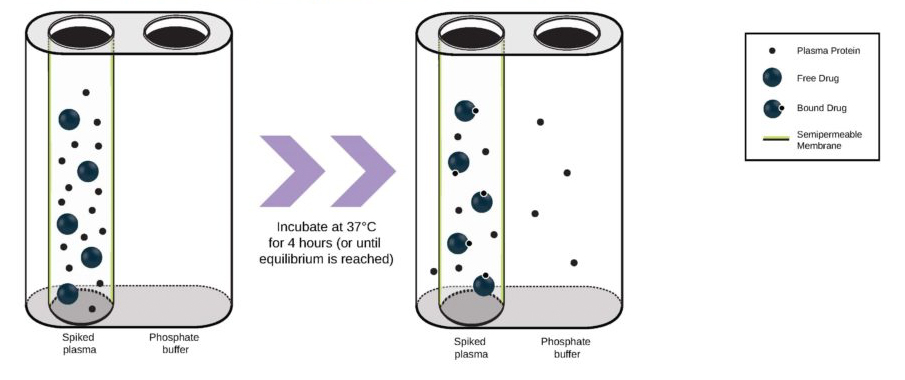
We’re pleased to announce that our preclinical laboratory has again earned a Full Accreditation from AAALAC International Council.
TD2 has held AAALAC accreditation since 2010, and we’re thrilled that the 2018 renewal continues that succession through 2021.
In their letter of notification, the AAALAC Council on Accreditation made special recognitions of the following features of our preclinical facility, staff and resources:
– Our administrative commitment to the program, along with the caliber of our documentation throughout it.
– The engagement of our Institutional Animal Care and Use Committee.
– The level of sanitation, cleanliness and organization in our facility.
– Our Occupational Health & Safety signage.
– Our tumor monitoring guideline.
– Our heating, ventilation and air conditioning monitoring system.
– Our storage and documentation practices for controlled substances.
We’re proud to support humane and responsible animal treatment throughout all ends of our scientific research, and we’re honored to continue this voluntary accreditation for years to come.
Want to learn more about our AAALAC-accredited preclinical lab? Ask us for a tour!