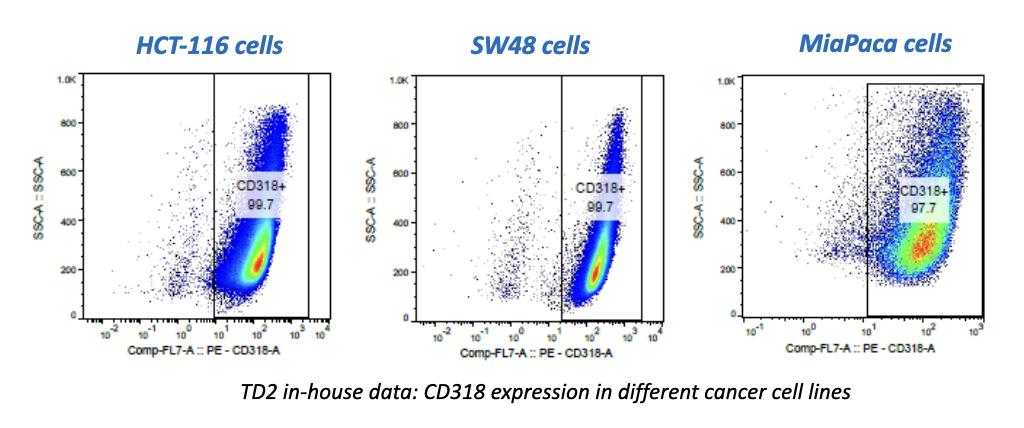
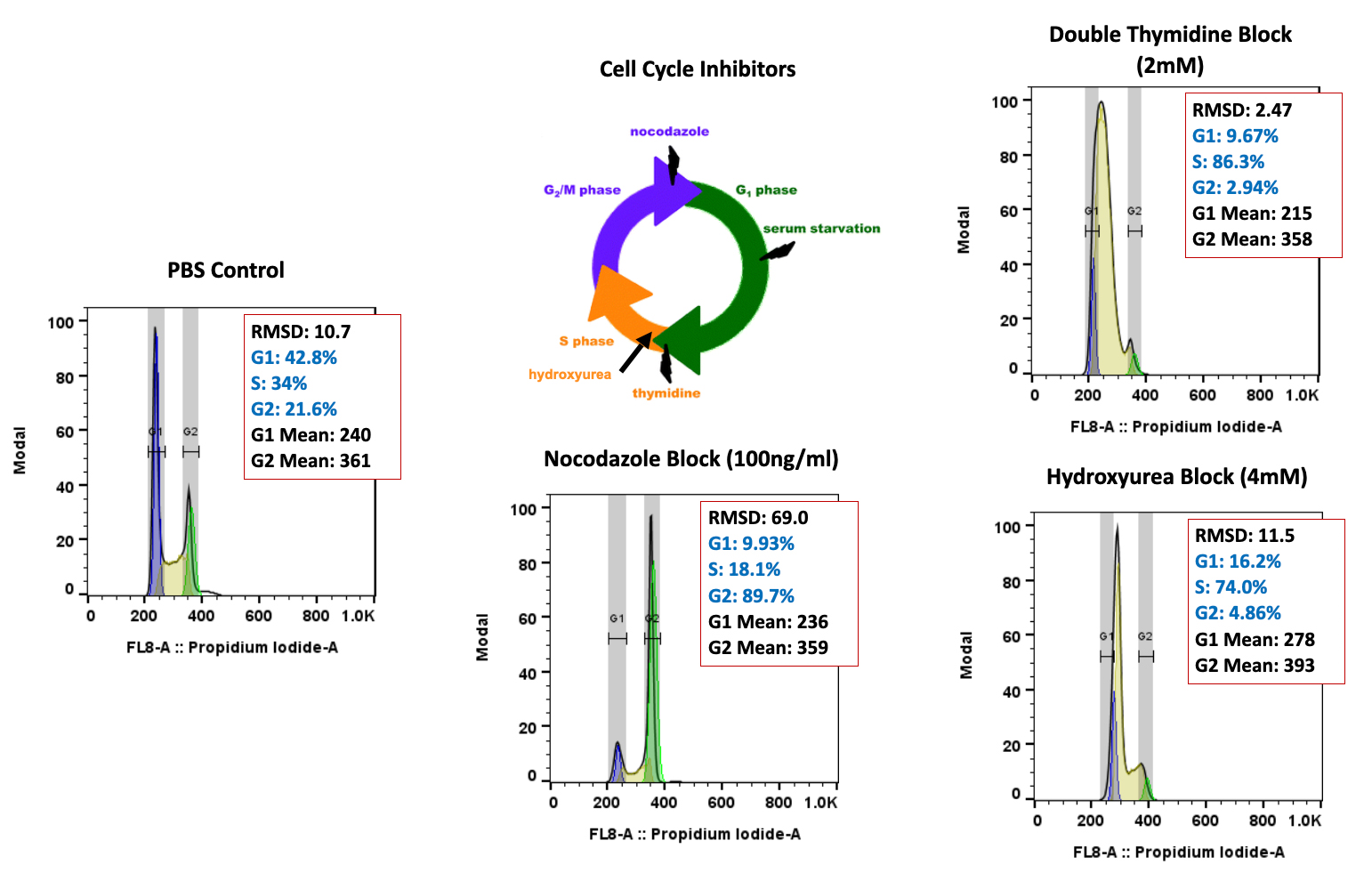
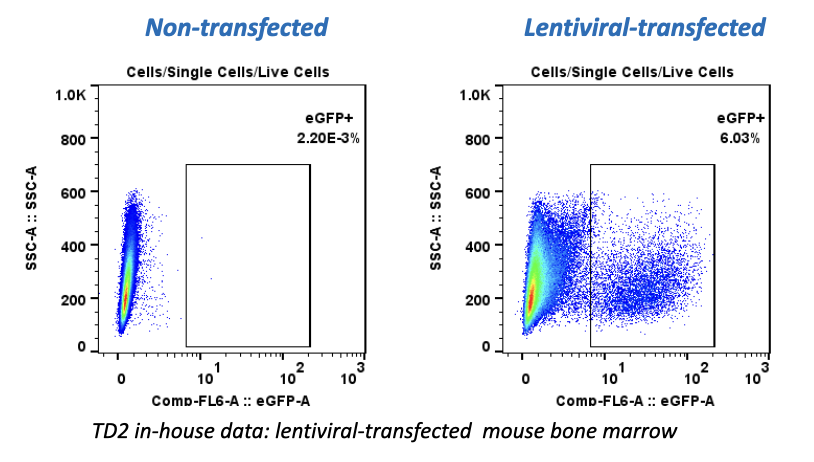
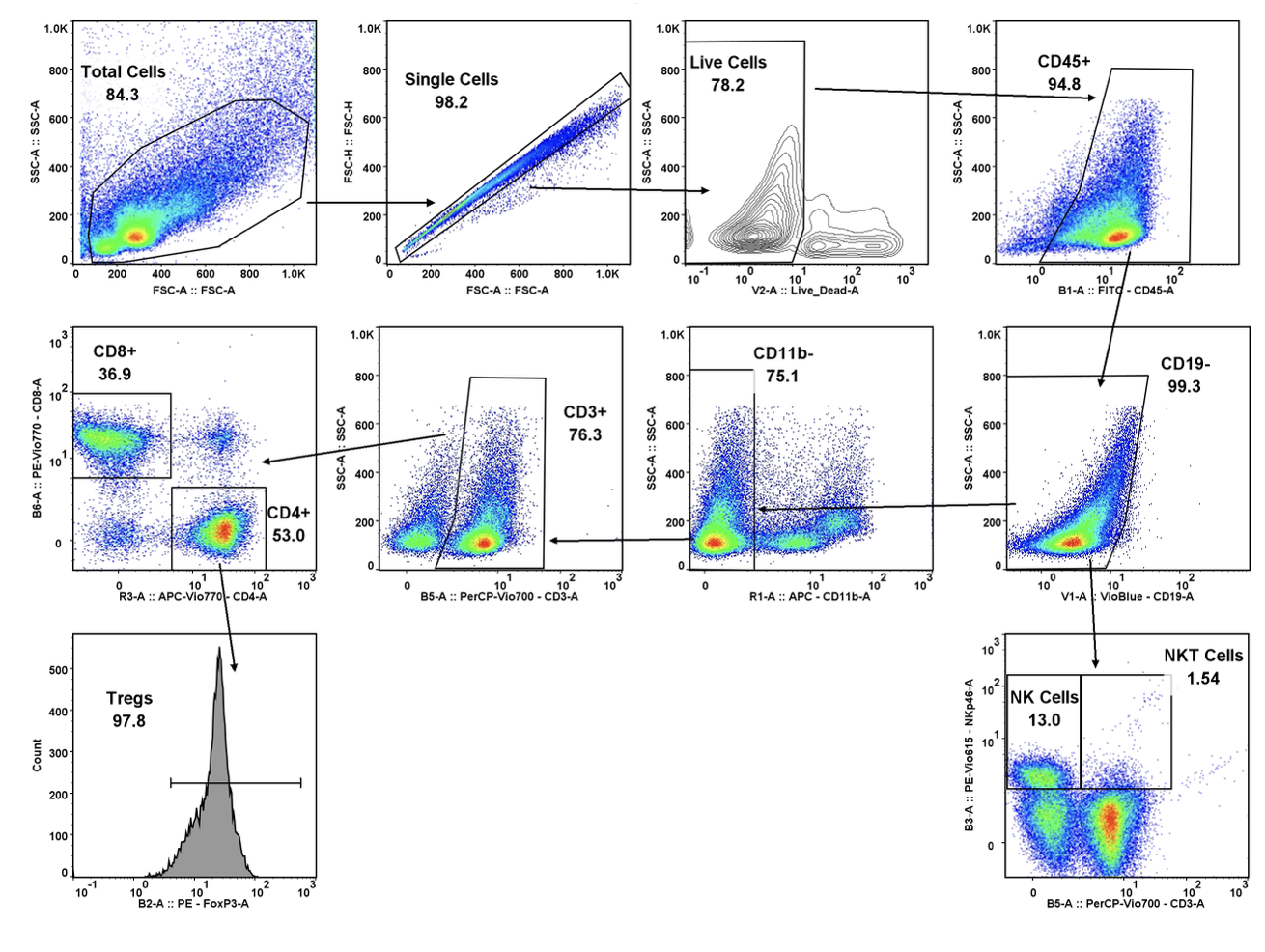
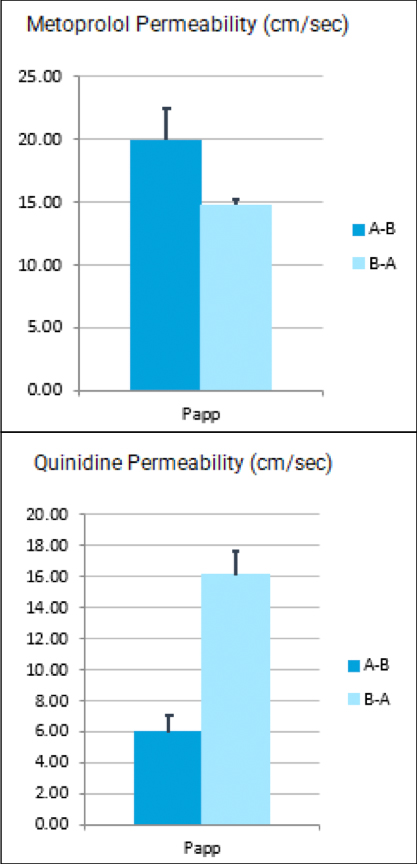
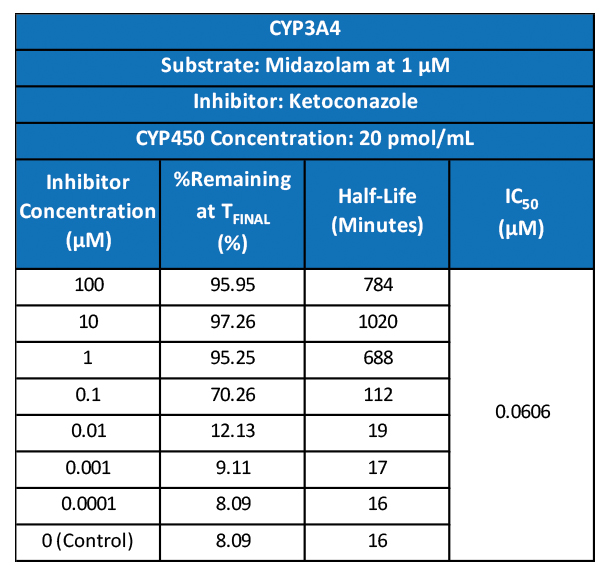
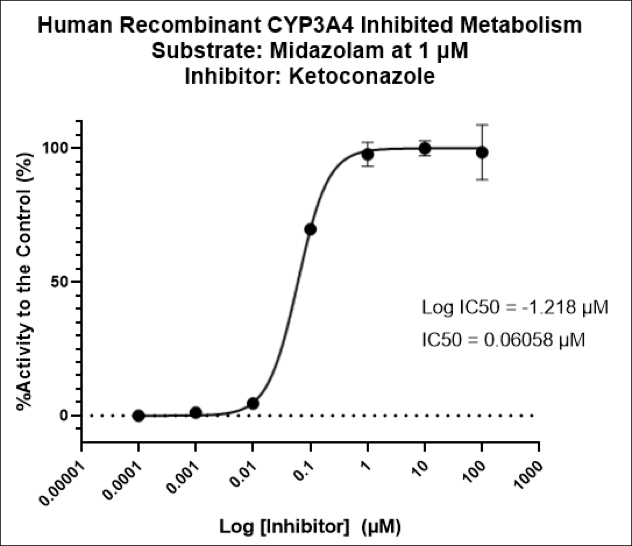
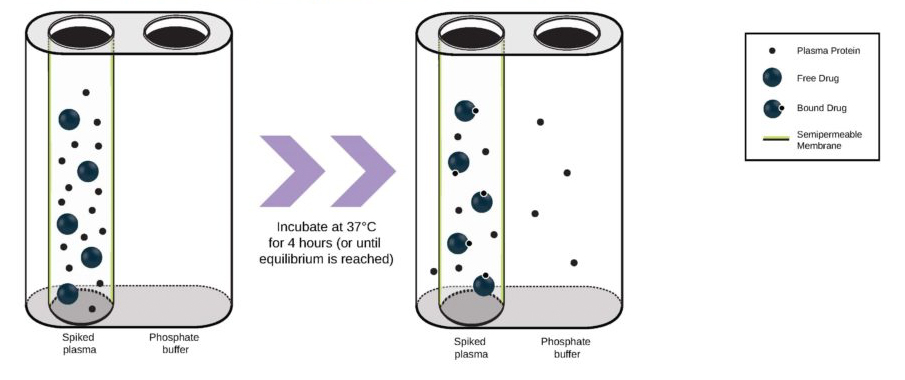
TGen Drug Development (TD2) and Imaging Endpoints have teamed up to enable the rapid development of anti-cancer drugs and deliver them faster to patients in need, the companies announced today.
“The highest quality medical imaging provided by Imaging Endpoints is one more weapon in TD2’s arsenal,” said Dr. Stephen Gately, President of TD2, which assists drug developers in navigating the regulatory maze involved in the successful completion of clinical trials and bringing new therapeutics to market. “Our relationship is meant to help combine the best available technologies.”
Imaging Endpoints will assist TD2 in the design and implementation of medical imaging analyses. Imaging Endpoints’ expertise in anatomic and functional imaging will allow drug developers to best incorporate imaging into their clinical trials: probing molecular drug targets, assessing biologic responses and evaluating drug effectiveness.
“We hope that, through our new relationship with TD2, we will be able to assist oncology drug developers to maximize their return on investment in medical imaging during the course of their early-phase clinical trials,” said Ron Korn, Medical Director of Imaging Endpoints. “Significant resources and time can be saved through wise and appropriate implementation of medical imaging.”