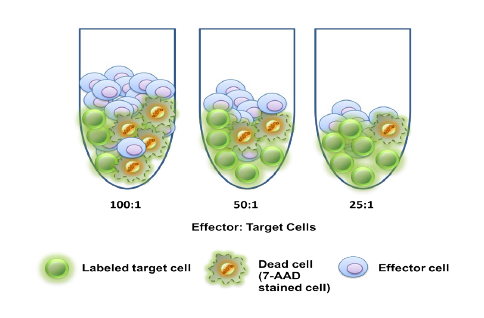
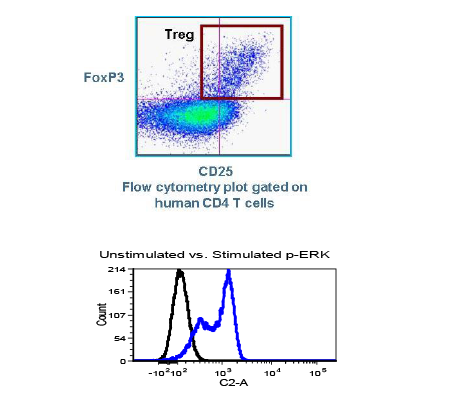
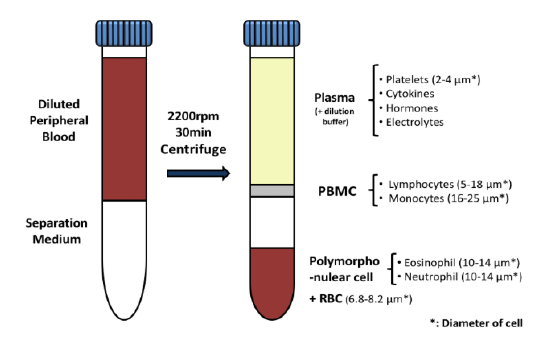
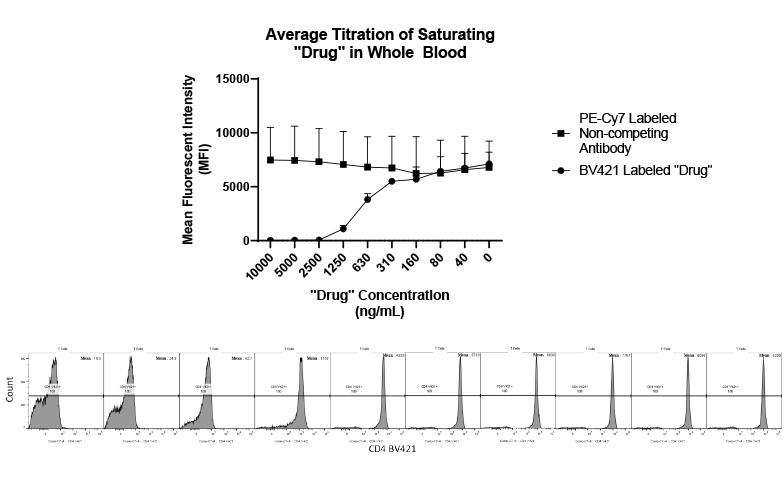
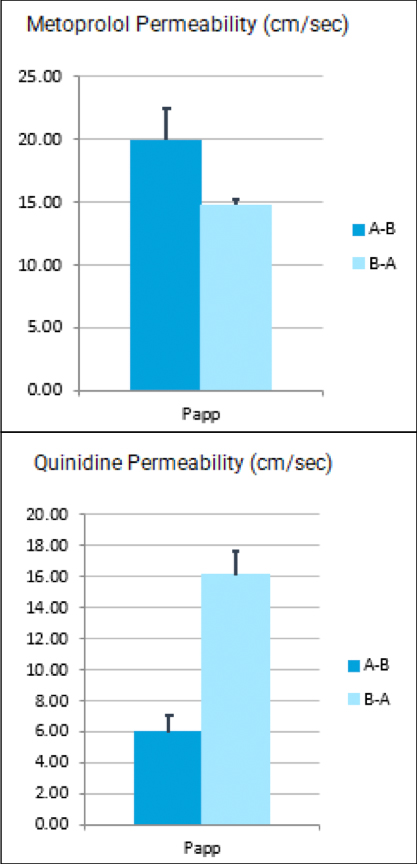
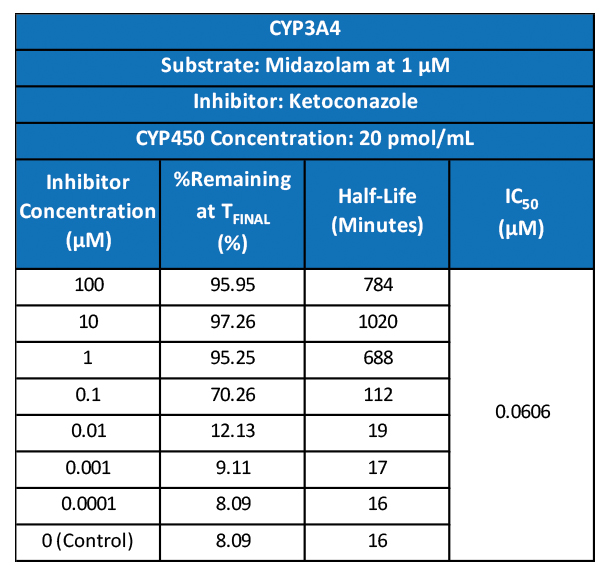
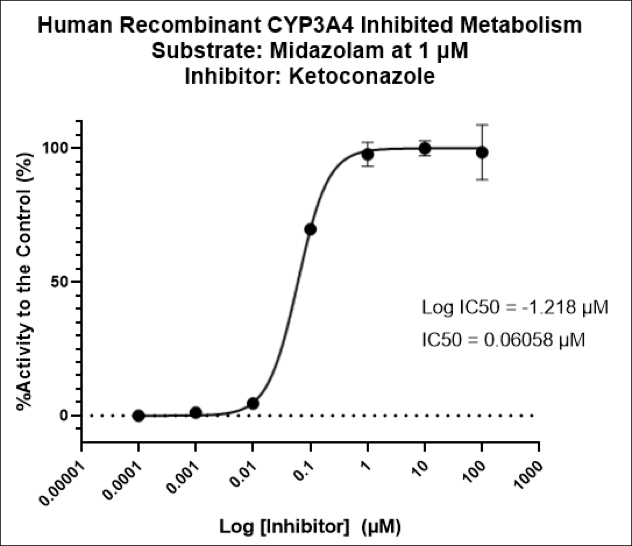
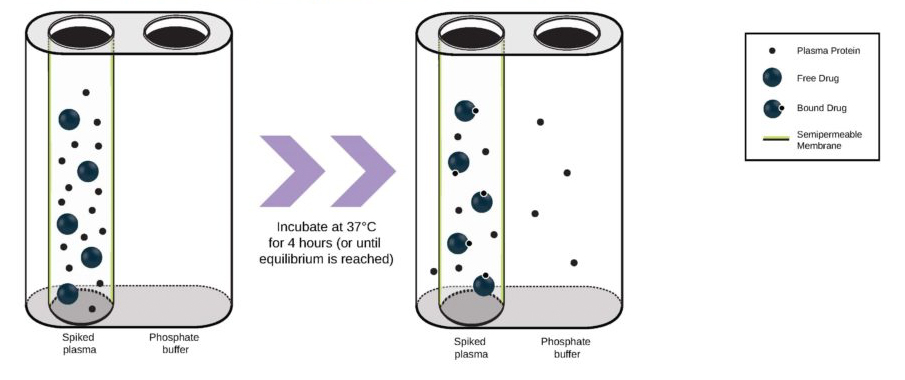
Used extensively in biological experiments and human clinical trials, randomization prevents both accidental and selection bias by eliminating sources of bias and producing comparable groups. Randomizing clinical trials also allows utilization of probability theory for expressing the likeliness of chance events as sources for differences in outcomes.
Randomization ensures each participant in a cancer clinical trial has the same chance of receiving one or more treatments as all other participants. In addition, randomization supports statistical methods applied to analyzing data generated from the trial while balancing groups regarding multiple unknown/known prognostic or confounding variables. Randomized experimentation in any field of scientific study is considered essential by ethical researchers for evaluating treatment efficacy.
In phase three clinical trials, randomization of subjects is mandatory. After assigning subjects to groups randomly, (a process usually involving a computer generator program), researchers give each group different treatments. For example, one group may receive the new treatment (the investigational group) while the group receives an older or standard treatment (the control group). Rarely are placebos used in clinical cancer trials. Several times during the trial’s progression, researchers will compare groups (via blood tests, urine samples, etc) and analyze the data to determine if the investigational group is benefiting from the new treatment.
Randomized Trial Investigating Effects of Anti-tumor Drug Nivolumab
A human immunoglobulin G4 programmed death–1 immune checkpoint inhibitor antibody, Nivolumab appears to restore T-cell immune activity. A randomized, phase II trial investigated the safety, antitumor effects and dose-response relationship of Nivolumab in subjects with metastatic renal cell carcinoma (mRCC). Authors of the study concluded that Nivolumab did demonstrate antitumor activity and had a “manageable safety profile” involving multiple doses in randomized mRCC patients. The safety and efficacy results supported by randomization allows progression of this research in a phase III setting.
Random assignment of cancer clinical trial participants to specific groups means the results are not affected by the subjectivity of human selection. For example, results of a cancer clinical trial would be invalid if it was discovered a researcher assigned subjects to groups according to their health status, i.e., the sicker patients went to the control group and the healthier patients went to the investigational group. Although bias like this may be unintentional, “subjective” randomization is likely to negate results and disqualify the trial.
By automatically controlling for any “lurking” variables, randomized cancer clinical trials allow researchers to control explanatory variable values. Therefore, if relationships between response and explanatory variables exist, there is evidence that the relationship is strictly causal, not predisposed by subjectivity and non-random events. In other words, if a new cancer treatment exhibits positive results following a randomized trial, that treatment warrants further investigation as a potentially effective anti-cancer treatment.
To learn more about the importance of randomization in cancer clinical trials, please contact us today.